
Georgia Valsartan Lawsuits
SERIOUS CASES. SERIOUS TRIAL LAWYERS. SERIOUS RESULTS.
$
600
MILLION
Whistleblower Case
$
42
MILLION
Medical Malpractice
$
40
MILLION
Medical Malpractice
$
25.9
MILLION
Medical Malpractice
$
18
MILLION
Automotive Products Liability
VIEW MORE
REPRESENTING CLIENTS IN ATLANTA, AUGUSTA, COLUMBUS, MACON, SAVANNAH, GAINESVILLE, LAWRENCEVILLE, DECATUR, JONESBORO & THROUGHOUT GEORGIA
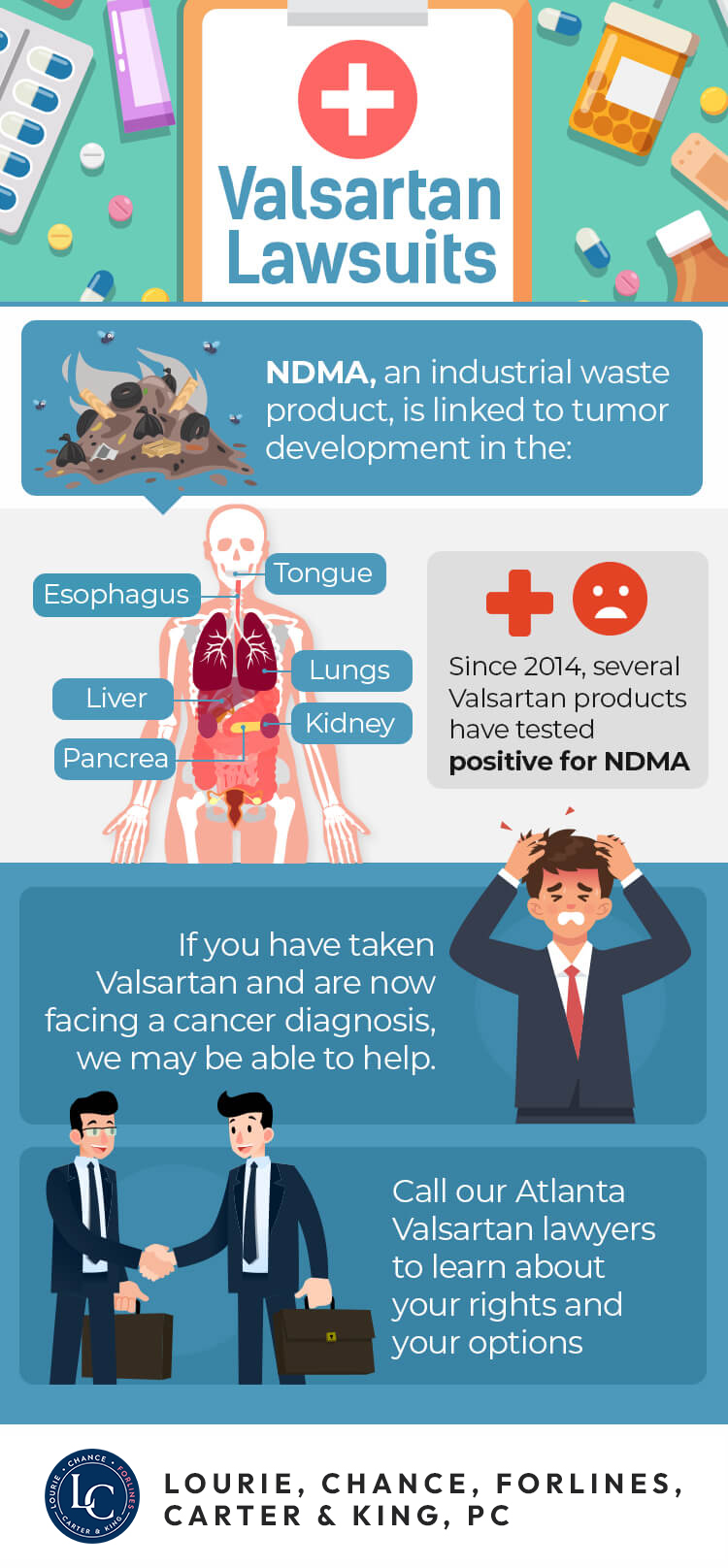
In July of 2018, the FDA issued a recall of valsartan products sold by Major Pharmaceuticals, Teva Pharmaceuticals dba Actavis LLC, and Prinston Pharmaceuticals dba Solco Healthcare LLC. Since that time, valsartan products sold by Camber Pharmaceuticals have been recalled and, in March of 2019, AurobindoPharma USA, Inc. and Acetris Health LLC issued their own voluntary recall for 39 lots of valsartan products. The issue with all of these is the presence of NDMA (N-nitrosodimethylamine), an organic chemical that is a suspected human carcinogen.
If you have taken a valsartan product and have been diagnosed with cancer, you may have cause to file suit. The dangerous medication attorneys at Chance, Forlines, Carter & King are here to review your case free of charge to help you understand your rights and your options. Call us at 404-760-7400 and schedule a consultation at our Atlanta office today.
WHAT IS VALSARTAN?
Valsartan is an angiotensin receptor blocker (ARB), designed to relax blood vessels to allow for an unobstructed flow of blood. Used to treat hypertension and as a method of reducing risks for heart attack, stroke, and kidney failure, valsartan is sold under several brand names including Novartis and Prexxartan.
Is the drug Valsartan being recalled?
Diovan, the parent company of Novartis, has not issued any recalls for Valsartan. However, many of the pharmaceutical companies that manufacture generic versions of the medication have issued recalls due to the presence of NDMA. Mylan Pharmaceuticals, a company that manufactures the active ingredient in Valsartan issued a voluntary recall of its products in November of 2019.
New generic versions of the drug were approved by the FDA in March of 2019. None of these have shown to have contaminants and none have, as of yet, been recalled.
What Are the Signs and Symptoms of NDMA Exposure?
On its own, valsartan may cause dizziness and lightheadedness with some patients reporting muscle weakness, fainting, and irregular heartbeat. Valsartan is not considered a dangerous medication when free of contaminants. However, there is evidence that many valsartan products manufactured since 2012 have been contaminated with NDMA, placing hundreds of thousands of people at risk for serious or fatal illness.
NMDA is linked to liver damage and reduced function of the kidney and lungs. According to the EPA, exposure to NDMA has also resulted in tumors in the liver, respiratory tract, kidney and blood vessels.
Is there a lawsuit against Valsartan?
There are currently multiple Valsartan lawsuits pending litigation. These suits allege that the presence of NDMA in Valsartan resulted in the development of cancerous tumors, liver and kidney failure, and other serious and potentially deadly problems. If you have been diagnosed with any of these conditions after taking Valsartan, you may have cause to file suit.
HAVE YOU BEEN INJURED?
If you have taken a valsartan product and are now facing a diagnosis of colorectal, stomach, liver, kidney, or bladder cancer, you may be entitled to compensation for medical treatment, any wages lost as a result of your diagnosis and subsequent treatment, and considerations for physical and emotional pain and suffering. The best way to learn if you have cause to file suit is through a private and confidential consultation at Chance, Forlines, Carter & King.
Our contaminated pharmaceutical product attorneys have decades of experience holding negligent corporations accountable and a long and proven track record of securing large settlements for victims of serious injury. If you believe your cancer is a result of taking a valsartan product, call our Atlanta office at 404-760-7400 to schedule a free consultation and learn more.
Located in Atlanta, our lawyers fight for victims of dangerous medications living throughout Georgia including the communities of Augusta, Decatur, Savannah, and Columbus.
PRACTICE AREAS
CASE RESULTS
TESTIMONIALS
"My siblings and I engaged Chance, Forlines, Carter & King, PC during an extremely stressful time in our lives, and we are thankful that they provided a warm working environment. We were highly impressed by their level of knowledge, their efficiency and their extreme professionalism. From our first contact to our very last conversation, we felt that our team at LCFCK genuinely cares about helping people."
V. FORD
READ MORE
